For over six decades, titanium has quietly revolutionized medicine—not by fighting the human body, but by becoming one with it. Unlike traditional metals that corrode or trigger rejection, titanium forms an invisible bond with living tissue, enabling everything from dental implants that last decades to hip replacements that restore mobility to millions. This unique harmony stems from titanium’s innate biocompatibility, a property so reliable that it has become the global standard for permanent medical implants. But not all titanium is created equal: only specific certified grades meet the rigorous demands of human implantation. This guide reveals the science behind titanium’s medical success, the precise grades approved for human use, and why surgeons trust it above all alternatives.
Why Titanium Works Inside the Human Body
Titanium’s unique ability to coexist with human tissue isn’t accidental—it’s a result of specific material properties validated by 60+ years of clinical use. Unlike most metals that corrode or trigger immune responses, titanium creates a perfect biological partnership:
- Self-Forming Oxide Layer: Upon exposure to air or bodily fluids, titanium instantly develops a 2-10 nanometer titanium dioxide (TiO₂) barrier. This inert film prevents metal ion release and shields tissues from direct contact with the metal.
- Osseointegration Capability: Bone cells (osteoblasts) adhere directly to titanium’s surface, growing microscopic “roots” into its microstructure. This creates mechanical bonds stronger than cemented alternatives—no fibrous tissue encapsulation occurs.
- Corrosion Resistance in Biological Environments: Titanium withstands constant exposure to chloride-rich body fluids (pH 7.4) at 37°C without degradation. Accelerated testing simulating 30+ years shows corrosion rates below 0.001 mm/year—effectively zero.
- Non-Toxic Degradation Products: Even if minimal wear occurs, titanium dioxide particles are biologically inert and cleared by macrophages without inflammatory response—unlike cobalt or nickel ions from other alloys.
Not All Titanium Grades Are Medical-Grade
Which Titanium Grades Are Approved for Human Implants?
While commercial titanium exists in 38 grades, only specific formulations meet stringent medical requirements. The FDA and global regulators recognize just three titanium types for permanent implants:
- ASTM F67 Grade 2 (UNS R50400): Commercially pure titanium with strict limits (O≤0.25%, Fe≤0.30%). Used for dental implants, cranial plates, and non-load-bearing devices. Its balance of strength and formability allows complex shapes without compromising biocompatibility.
- ASTM F67 Grade 4 (UNS R50700): Higher-strength CP titanium (O≤0.40%) for applications requiring 500+ MPa yield strength. Common in trauma fixation plates and dental abutments where thin cross-sections are needed.
- ASTM F136 Ti-6Al-4V ELI (Grade 23, UNS R56401): The gold standard for load-bearing implants. “ELI” (Extra Low Interstitial) controls oxygen (≤0.13%), iron (≤0.25%), and hydrogen (≤0.0125%) to prevent embrittlement. Used in hip stems, spinal rods, and knee replacements where fatigue resistance is critical.
Critical Quality Requirements
Medical-grade titanium must pass:
- Hydrogen content testing per ASTM F1472 (<0.0125%)
- Inclusion rating per ASTM E45 (maximum 1.5 severity)
- Ultrasonic inspection per ASTM E2375 (zero internal voids)
- Surface roughness validation (Ra ≤0.8 μm for bone-contact surfaces)
Industrial grades (like ASTM B348) lack these controls and must never be used for implants—despite identical nominal compositions.
Titanium Safety
Over 50 million titanium implants have been placed worldwide since 1970. The data reveals remarkable safety:
- Allergy Incidence: Less than 0.6% of patients show sensitivity to pure titanium (vs. 10-15% for nickel-containing stainless steel). Most reported “titanium allergies” trace to aluminum/vanadium in non-ELI alloys or surface contaminants.
- Metal Toxicity: Zero evidence of titanium accumulation in organs. Blood levels in implant patients average 1.2 μg/L—below natural background levels (2.0 μg/L) in humans. Unlike heavy metals, titanium ions aren’t metabolized or stored.
- MRI Compatibility: ASTM F67/F136 titanium is non-ferromagnetic. Patients safely undergo 3T MRI scans with no heating, movement, or significant image artifacts.
- Long-Term Survival: 95% of titanium hip replacements function after 15 years (vs. 80% for ceramic alternatives). Dental implants show 98% 10-year survival in meta-analyses of 47 studies.
Critical Medical Applications of Titanium
Orthopedic Implants:
- Hip/knee replacements with porous-coated surfaces for bone ingrowth
- Spinal fusion cages that maintain alignment while new bone forms
- Bone plates and screws that resist loosening in fracture healing

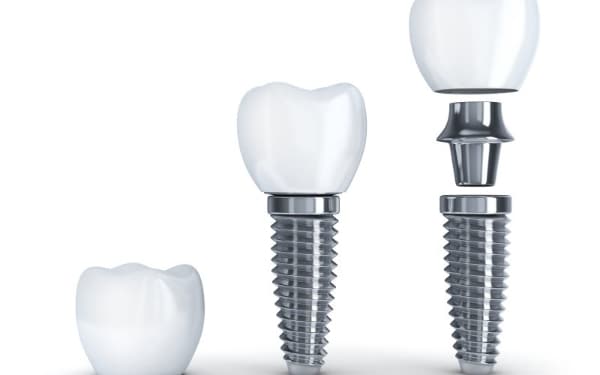
Dental Systems:
- Endosteal implants that replace tooth roots with 98% success rate
- Custom abutments matching gum contours for natural appearance
- Temporary anchorage devices (TADs) for orthodontic correction
Cardiovascular Devices:
- Pacemaker and defibrillator housings that withstand 100,000+ heartbeat cycles
- Occlusion devices for congenital heart defects
- Stent components in peripheral vascular applications

Surgical Tools:
- Reusable instruments that survive 1,000+ autoclave cycles without corrosion
- MRI-compatible surgical handles for intraoperative imaging
- Lightweight forceps and retractors reducing surgeon fatigue

Emerging Frontiers:
- 3D-printed trabecular structures mimicking bone density for cranial reconstruction
- Drug-eluting titanium surfaces releasing antibiotics at infection sites
- Neural electrode arrays for brain-computer interfaces (BCIs)

Questions fréquemment posées
Q: Can titanium implants cause metal poisoning or systemic toxicity?
A: No evidence exists after 60+ years of use. Titanium ions aren’t metabolized like heavy metals. Serum titanium levels in implant patients average 1.2 µg/L—below the 2.0 µg/L natural background in humans (FDA Guidance Document, 2025).
Q: What about aluminum/vanadium in titanium alloys?
A: Standard Grade 5 (Ti-6Al-4V) isn’t medical-grade. ASTM F136 requires ELI (Extra-Low Interstitial) version with strict limits: Al ≤6.0%, V ≤4.0%, and oxygen ≤0.13%. No peer-reviewed study links ASTM F136 implants to aluminum neurotoxicity.
Conclusion
Titanium remains unmatched for permanent medical implants due to its unique combination of biocompatibility, strength, and corrosion resistance. Strict material standards (ASTM F67/F136) ensure only properly processed titanium enters the human body—never industrial grades.
Daxun Alloy Co. Ltd. is a supplier with years of experience in the titanium alloy industry. We supply fully certified medical-grade titanium alloys compliant with ASTM F67/F136 and ISO 5832 standards. Contact our professional engineers today for the best solutions.




